
In this video we are going to determine the polarity of Oxygen
Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7.
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
The Lewis structure indicates that each Cl Cl atom has three pairs of electrons that are not used in bonding (called lone pairs ) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond .
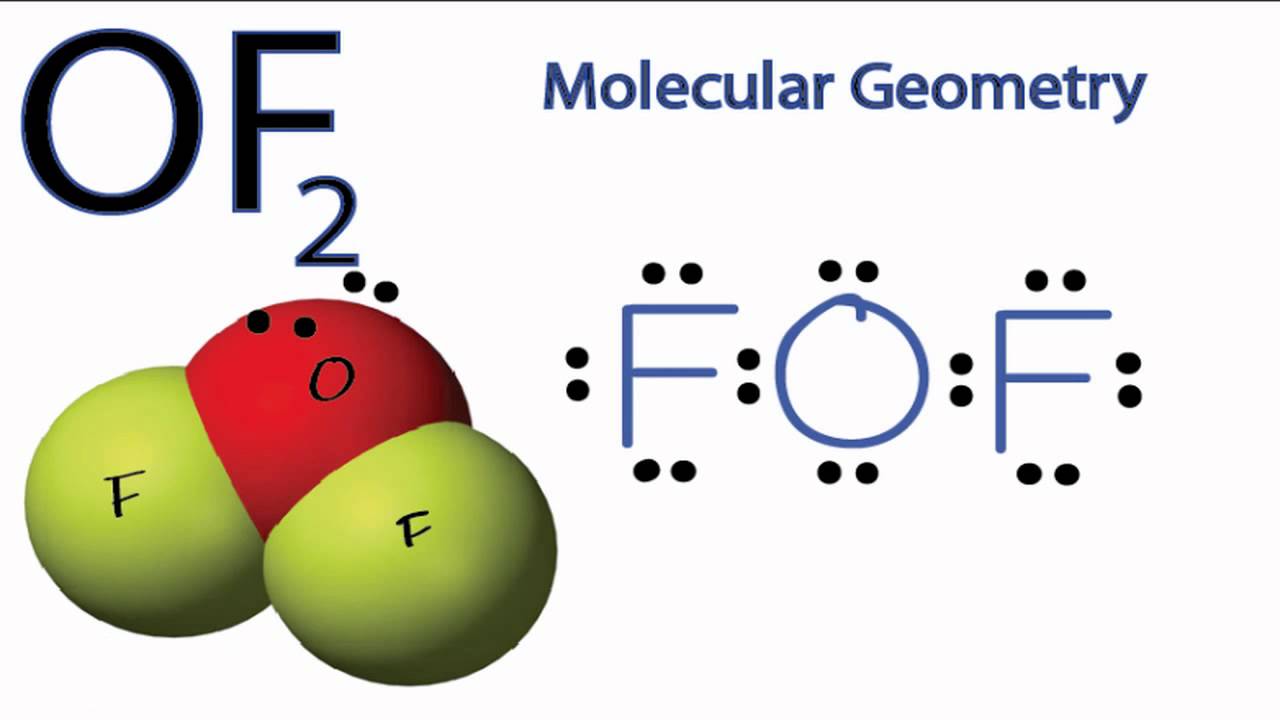
OF2 Molecular Geometry YouTube
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is therefore. formalcharge(N) = 5 −(0 + 8 2) = 0.
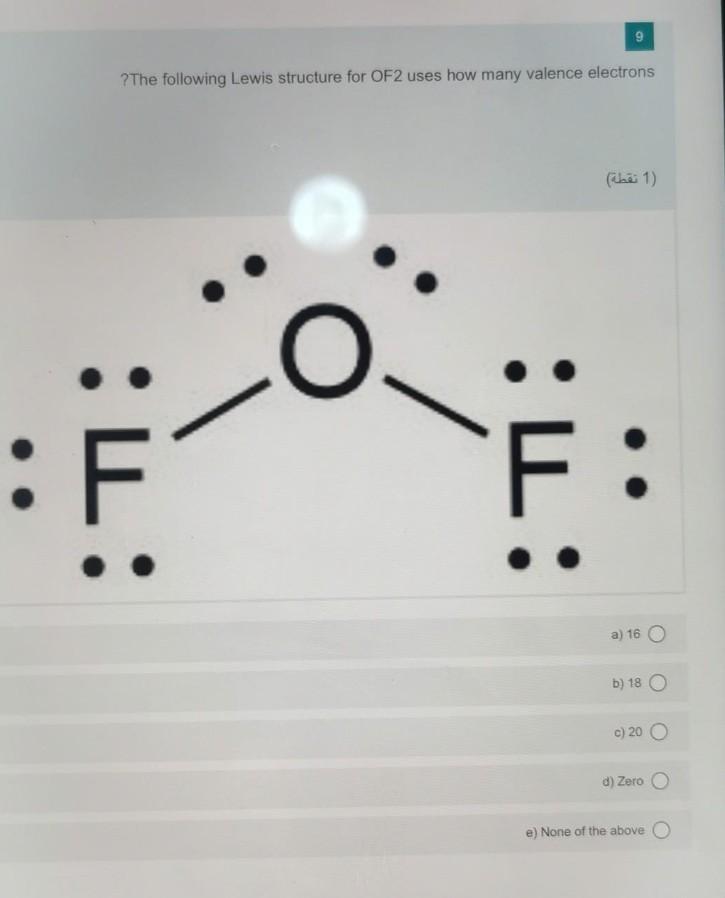
Solved 9 ?The following Lewis structure for OF2 uses how
The information on this page is fact-checked. OF 2 Lewis structure. OF 2 (oxygen difluoride) has one oxygen atom and two fluorine atoms. In the OF 2 Lewis structure, there are two single bonds around the oxygen atom, with two fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the oxygen atom has two lone pairs.

H2S Lewis Structure explanation Molecular Geometry, Web Story, Science
-the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for O 2 ( Oxygen Difluoride) Oxygen difluoride (OF 2) isn't too tough of a Lewis structure since it only has single bonds. There are 20 valence electrons available for the Lewis structure for OF 2. So you'll be busy drawing some dots.

Draw the Lewis structures of the given molecules. Include lone pairs on
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

OF2 Lewis Structure How to Draw the Lewis Structure for OF2 YouTube
A step-by-step explanation of how to draw the OF2 Lewis Dot Structure (Oxygen difluoride). For the OF2 structure use the periodic table to find the total number of valence electrons for.
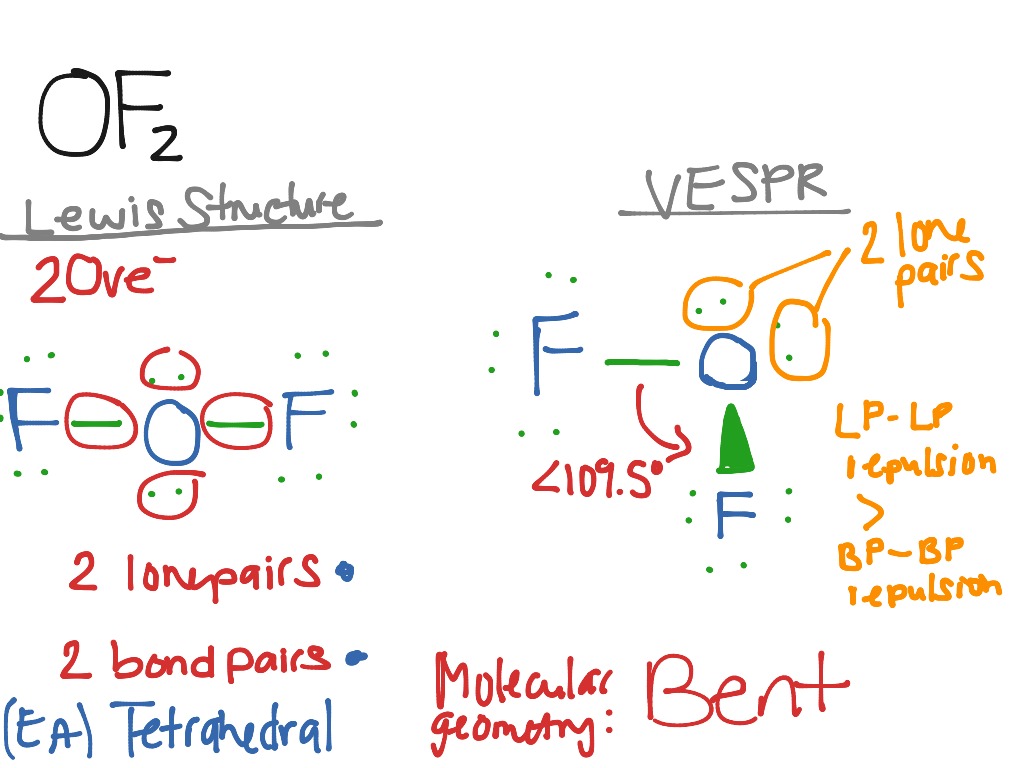
OF2 Science, Chemistry, VESPR ShowMe
For \(\ce{OF2}\), we had 16 electrons remaining in Step 3, and we placed 12, leaving 4 to be placed on the central atom:. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Two electrons.
OF2 lewis structure, molecular geometry, hybridization and bond angle
= 20 valence electrons Thus, there are 20 valence electrons available for OF2 that help the atoms to form bonds. OF2 Lewis Structure Now that we know the total number of valence electrons for OF2, we can make the Lewis dot structure of OF2.
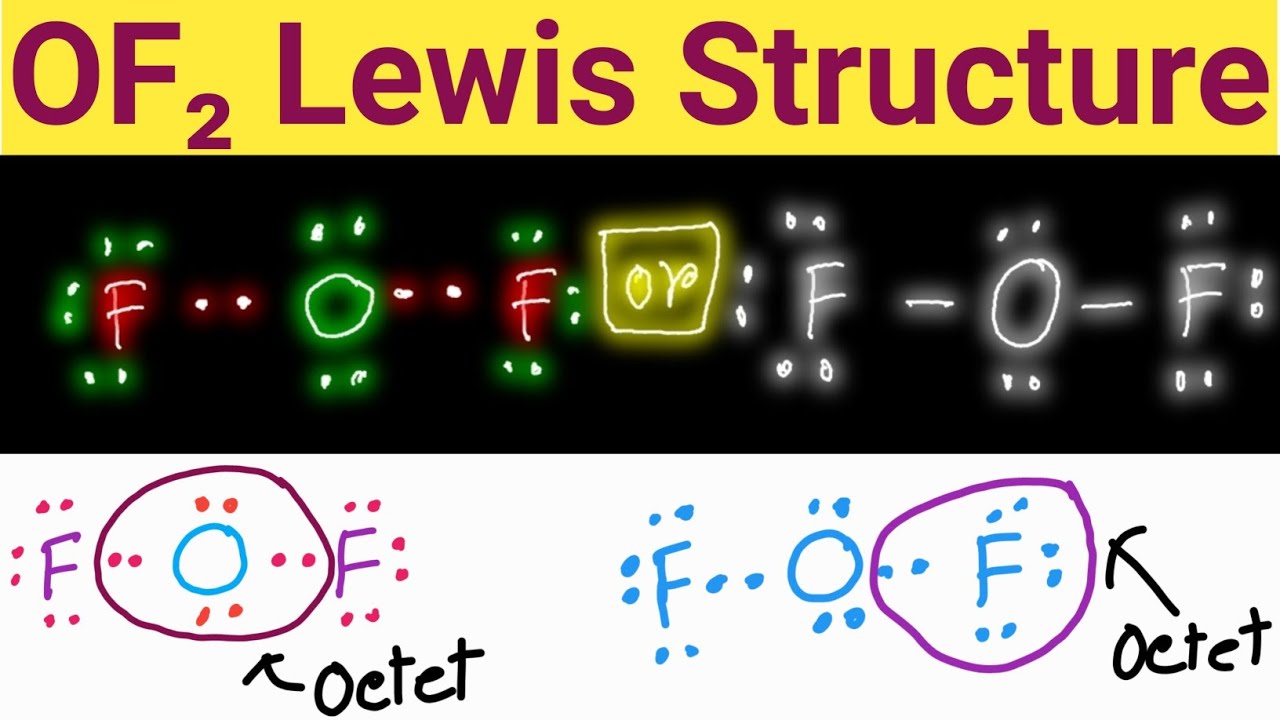
OF2 Lewis Structure Lewis Dot Structure for OF2 Oxygen Difluoride
To create the Lewis structure of OF2, follow these steps: 1. Counting Valence Electrons Begin by counting the total valence electrons in the molecule. In OF2, we have: Oxygen (O) - 6 valence electrons Fluorine (F) - 7 valence electrons each (2 atoms) Total valence electrons = 6 + 7*2 = 20 2. Placing the Least Electronegative Atom in the Center
[Solved] Draw a Lewis structure for each molecular compound a. OF2 b
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
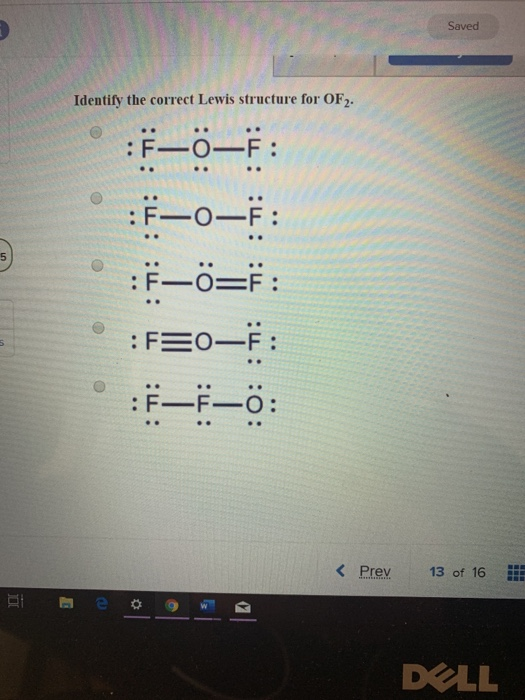
Solved Saved Identify the correct Lewis structure for OF2.
OF2, aka Oxygen Difluoride, is a MOLECULAR compound.Oxygen brings 6 electrons, each Fluorine brings 7 electrons, that's 20 electrons total.That's enough for.

Answer please! In OF2, the total number of bond pairs and lone pairs of
Watch on Steps of drawing OF2 lewis structure Step 1: Find the total valence electrons in OF2 molecule In order to find the total valence electrons in OF2 (oxygen difluoride) molecule, first of all you should know the valence electrons present in oxygen atom as well as fluorine atom.
[Solved] What is the correct Lewis structure for oxygen difluoride
Lewis structure of OF2 contains two single bonds between the Oxygen (O) atom and each Fluorine (F) atom. The Oxygen atom (O) is at the center and it is surrounded by 2 Fluorine atoms (F). The Oxygen atom has 2 lone pairs and both the Fluorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.
28+ Of2 Lewis Structure Molecular Geometry Gif GM
How to Draw Lewis structure for OF2 Oxygen difluorideLewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Subs.

What is the Lewis dot structure of \ce{OF2}? Quizlet
The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF 6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Two electrons remain, and this lone pair is placed on the Xe atom: Exercise 4.2. 2: interhalogens.