[Solved] Draw the Lewis structure for CF4 , and indicate both the
CF4 lewis structure has a Carbon atom (C) at the center which is surrounded by four Fluorine atoms (F). There are 4 single bonds between the Carbon atom (C) and each Fluorine atom (F). There are 3 lone pairs on all the four Fluorine atoms (F).
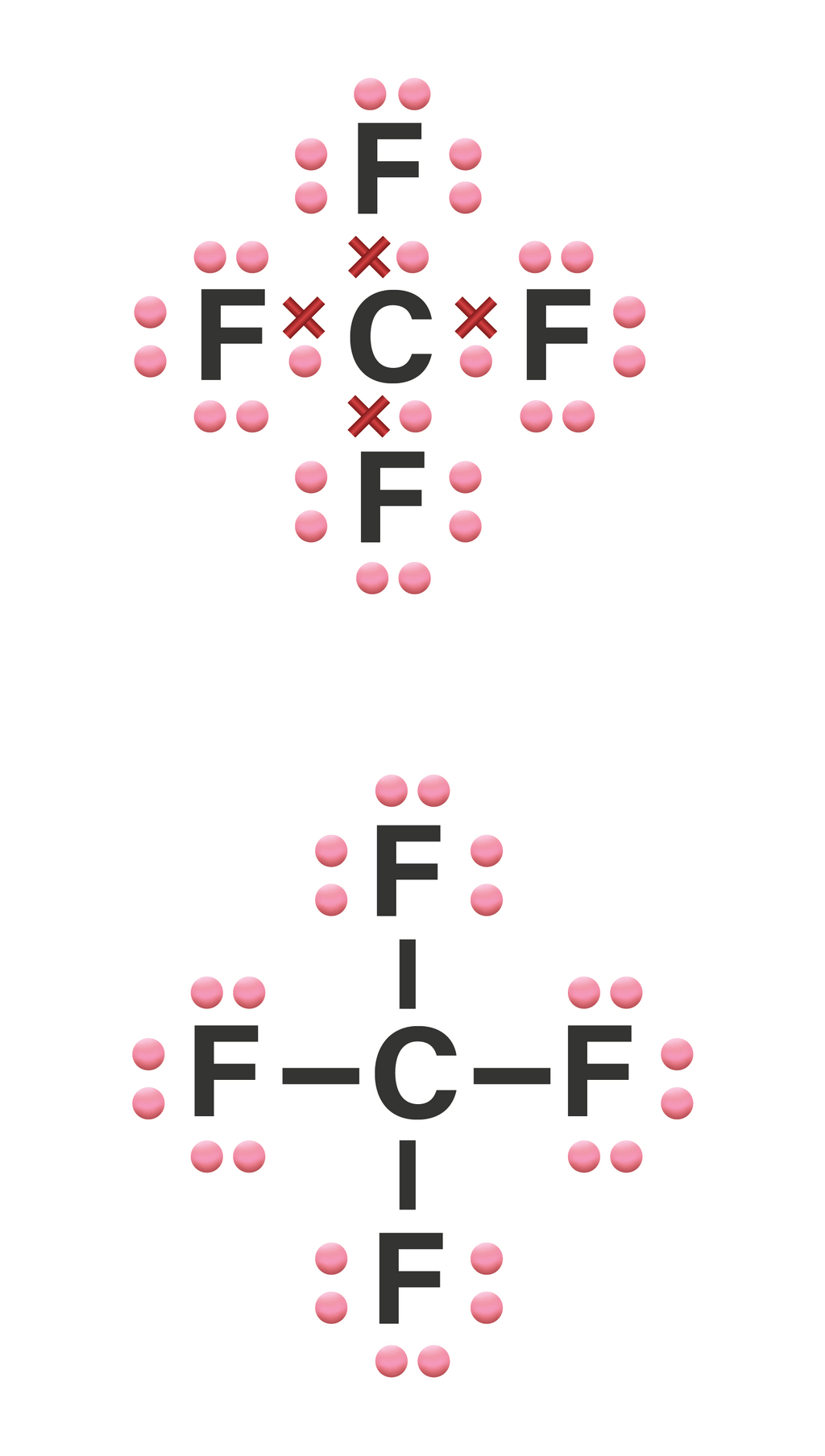
Tuliskan struktur Lewis dari molekul CF4!...
Lewis structure of CF4 contains four single bonds between the Carbon (C) atom and each Fluorine (F) atom. The Carbon atom (C) is at the center and it is surrounded by 4 Fluorine atoms (F). The Carbon atom does not have a lone pair while all four fluorine atoms have three lone pairs each.

Draw the geometry of following compounds (i) SF4 (ii) xeF4 Chemistry
What is the Lewis dot structure for CF4? Question: What is the Lewis dot structure for C F 4? The Lewis Dot Structure: The Lewis dot structure for any molecule can be found by.

Pin on Geometry Of Molecules
Now, let's learn to draw the Lewis structure of carbon tetrafluoride (CF4) with steps: Step 1: Find the total number of valence electrons each carbon tetrafluoride (CF4) molecule has: It is 32 as 4 are coming from the carbon atom and 7 are coming from each fluorine atom.
Lewis Structure Cf4
In the CF 4 Lewis structure, there are four single bonds around the carbon atom, with four fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs. How to Draw The Lewis Structure for CF4 (Carbon Tetrafluoride) Watch on Contents Steps #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms

CF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
The formula of CF4 molecular hybridization is as follows: No. Hyb of CF4 = N.A (C-F bonds) + L.P (C) No. Hy of CF4= the number of hybridizations of CF4. Number of C-Fbonds = N.A (C-F bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CF4 molecule.

How to draw a CF4 Lewis Structure? Science Education and Tutorials
What is the Lewis structure of [//substance:cf4//]? Natural Language Math Input Extended Keyboard Examples Random Assuming completion date | Use opening date instead Using closest Wolfram|Alpha interpretation: Lewis structure Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals.
Carbon Tetrafluoride Formula Answered What Is The Chemical Formula Of
A three-step approach for drawing the CF4 Lewis Structure can be used. The first step is to sketch the Lewis structure of the CF4 molecule, to add valence electron around the carbon atom; the second step is to valence electron to the fluorine atom, and the final step is to combine the step1 and step2 to get the CCl4 Lewis Structure.

Lewis Structure Carbon Tetrafluoride Cf4 Stock Vector (Royalty Free
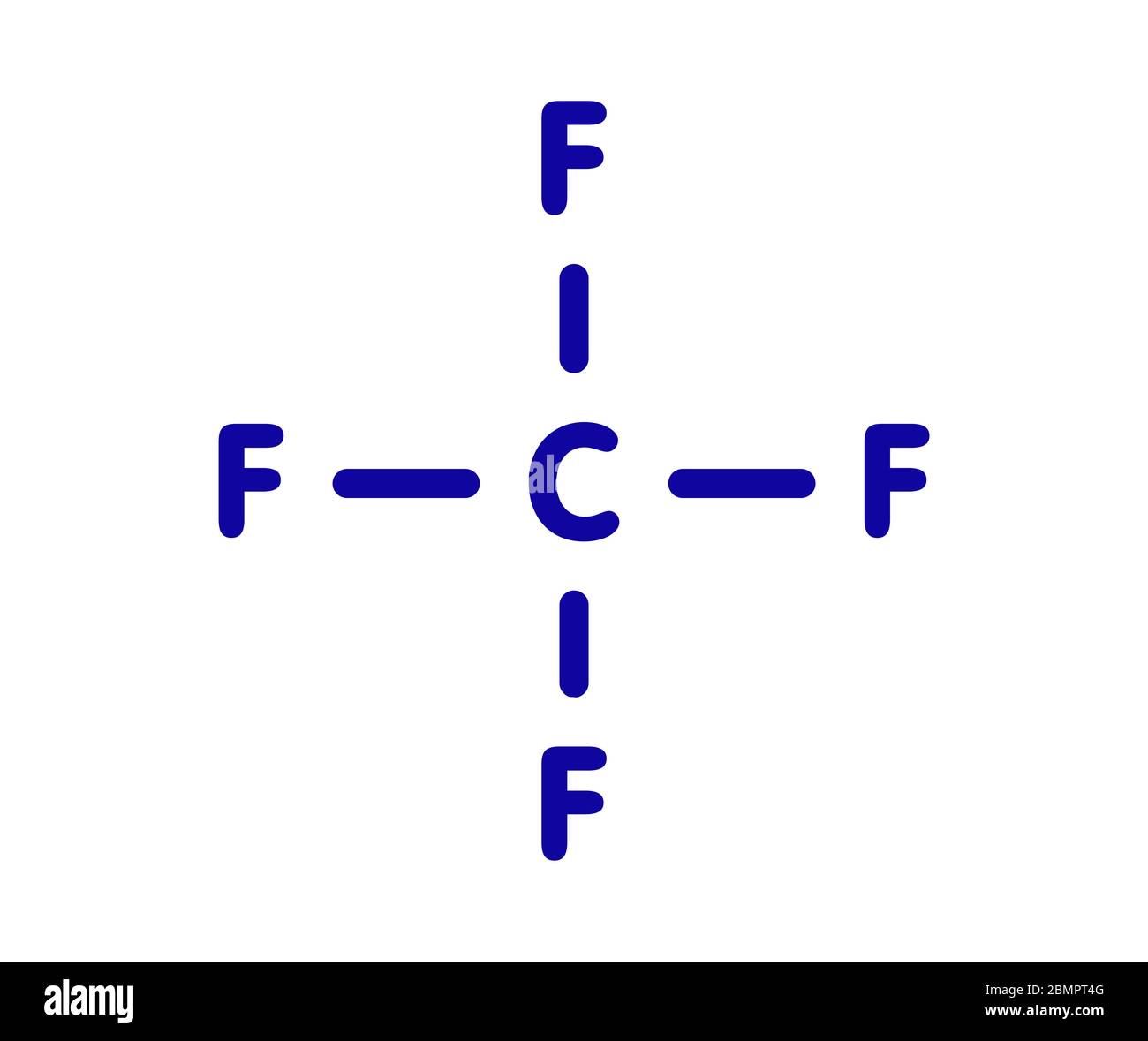
Example - Two Different Lewis Structures: CF4 (OpenChem)

CF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
How to Draw a Lewis Structure for CF4? Carbon tetrafluorideLewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2c.
41+ Cf4 Molecular Geometry Bond Angles Tips GM
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

How to draw a CF4 Lewis Structure? Science Education and Tutorials
122 15K views 3 years ago An explanation of the molecular geometry for the CF4 (Carbon tetrafluoride) including a description of the CF4 bond angles. The electron geometry for the Carbon.
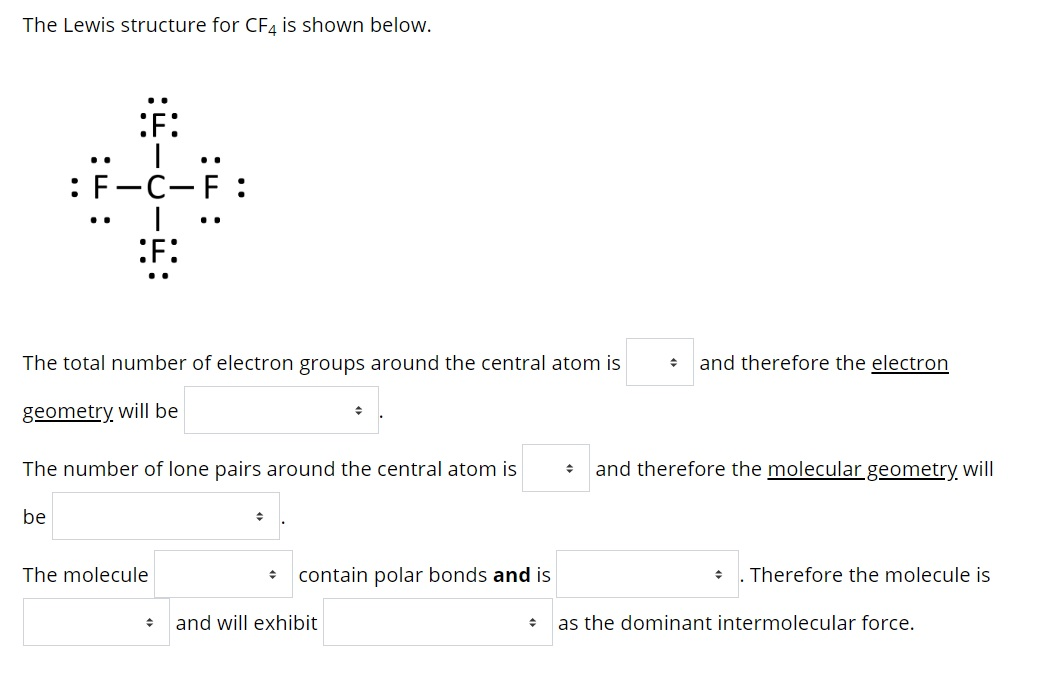
Solved The Lewis structure for CF4 is shown below. F
Steps for Writing Lewis Structures. Calculate the sum of the valence electrons in the molecule. 1 C atom = 1 × 4 = 4 valence e -. 1 O atom = 1 × 6 = 6 valence e -. 2 Cl atoms = 2 × 7 = 14 valence e -. sum of valence e - = 24 valence e -. Construct a skeleton structure for the molecule. C is the central atom since it makes the most.
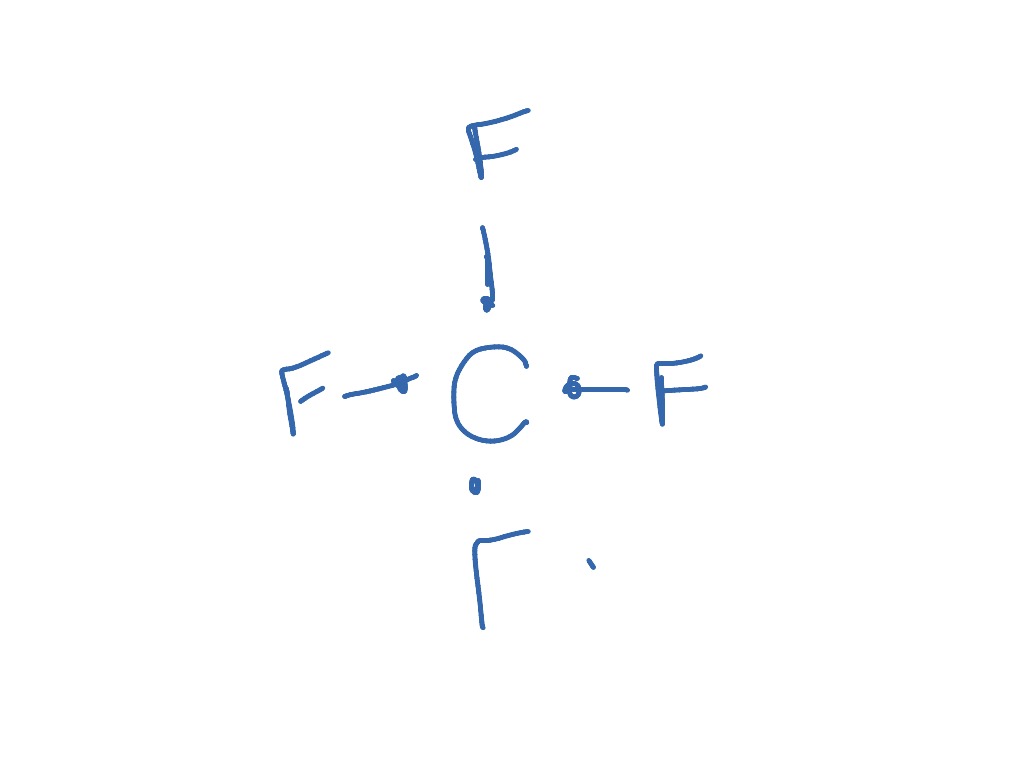
Cf4 Science ShowMe
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

CF4 Lewis Structure Lewis Dot Structure for CF4Lewis Structure of
Written by Priyanka in Lewis Structure The chemical formula CF4 represents Carbon Tetrafluoride. It is also known as a Tetrafluoromethane (IUPAC name) and R-14 (owing to its use as a refrigerant). CF4 is the simplest perfluorocarbon (Hydrocarbons in which C-F bonds have replaced all the C-H bonds).

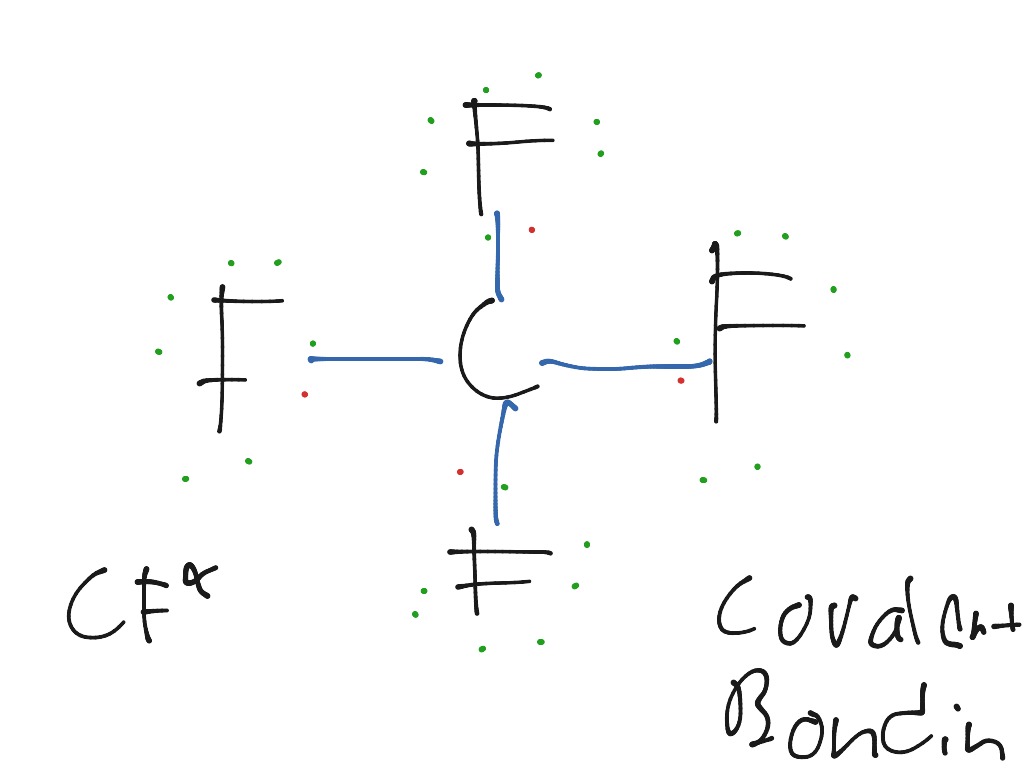
Carbon tetrafluoride (CF4) is a potent greenhouse gas. Draw a Lewis dot
How to Draw The Lewis Structure for CF4 (Carbon Tetrafluoride) Watch on So from the above diagram we have come to know that the CF4 molecule has four C-F bonds. Now in the next step we have to check whether these four C-F bonds are polar or nonpolar. Step #2: Check whether individual bonds are polar or nonpolar